Today we have the second guest post in our colloidal silver series.
This month we will be looking at what kind of colloidal silver should one be looking for?
It is interesting to note the different kinds of products with the name colloidal silver, but which are not colloidal silver in their entirety or at all, that are marketed and sold as colloidal silver at stores and online.
So here are the characteristics we should look for before buying or making colloidal silver:
The Super-Tyndall Effect
As we mentioned in the last article under the question, What About The Tyndall Effect And Silver?, silver in a molecular suspension is usually toxic. On the other hand, silver in a microheterogeneous suspension can cause argyria, a condition where silver is deposited as a heavy metal and a permanent discoloration may occur. In either case, too small particles or too large particles, there is a risk.
As explained before, What About The Tyndall Effect And Silver?
 A nineteenth century scientist named John Tyndall was the first to identify a narrow band of liquid suspensions in which light was scattered, known as the Tyndall effect. These suspensions that scatter the light are known as colloids. A weak Tyndall effect suggests that only a small portion of the suspension is colloidal. The stronger the Tyndall effect, the more of the suspension is colloidal. Some colloids exhibit such a profound Tyndall effect, even normal room lighting is adequate to observe the effect. In these cases the suspension is likely almost entirely colloidal. These colloids of silver exhibit a super-Tyndall effect and are considered the highest quality.
A nineteenth century scientist named John Tyndall was the first to identify a narrow band of liquid suspensions in which light was scattered, known as the Tyndall effect. These suspensions that scatter the light are known as colloids. A weak Tyndall effect suggests that only a small portion of the suspension is colloidal. The stronger the Tyndall effect, the more of the suspension is colloidal. Some colloids exhibit such a profound Tyndall effect, even normal room lighting is adequate to observe the effect. In these cases the suspension is likely almost entirely colloidal. These colloids of silver exhibit a super-Tyndall effect and are considered the highest quality.
The picture above shows the Tyndall effect. Notice the glow from the bottle on the right compared to the other silver solution.
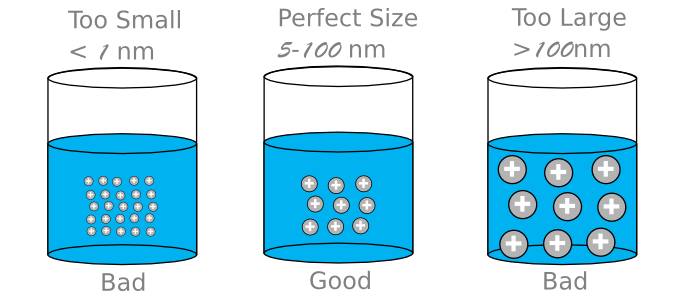
Charged Particles and Size
High quality colloids of silver owe their stability to like-electrically-charged nanoparticles. Such charged nanoparticles repel each other like same-pole magnets. If the size (mass) of the particles is small enough, the pull of gravity will be less than the repelling force and the particles can remain suspended indefinitely. If the particle size is too large, the pull of gravity will be greater than the repelling force and the particles will eventually settle to the bottom of the container. Homemade and other low-grade colloidal silvers with nanoparticles that are too large and use stabilizers (such as gelatin, protein, starch, fruit juices, etc.) to slow the settling in order to appear to be stable fall in this category. Don’t be fooled by this slight-of-hand practice, remember argyria can develop from ingesting large quantities of these larger nanoparticles of silver. On the other hand, other low-grade colloidal silvers are made mostly of small nanoparticles that are so small the Super-Tyndall effect is weak or non-existent, remember these nanoparticles are too small and therefore toxic.
Concentration Window
In order to avoid silver toxicity as much as possible, keeping the colloidal silver concentration at or below 30 ppm seems to work the best. On the other hand, colloidal silver concentrations from 15 ppm and above are 100% effective at killing all the members of organisms being studied. Below 15 ppm, not all of the members of the organisms are killed and thus are able to recover and proliferate. Therefore, the window of colloidal silver concentrations from 15 ppm to 30 ppm seems to be the best because these concentrations avoid silver toxicity while retaining the 100% effectiveness.
Color
Properly made colloidal silver has certain color which is affected by the nanoparticles’ size and concentration. This color can be seen without the need of expensive equipment. When the nanoparticles are too small or are in very low concentrations, they do not show any color (they look clear like water). As the size of the nanoparticles or their concentration increases, a light yellow color can be seen. This yellow color intensifies until the colloidal silver solution looks orange-red as light passes through it and grayish-green on the outside as light reflects on the silver solution. Larger than 80 nm nanoparticles at large concentrations will look mostly green. So a light yellow to yellow colloidal silver with a concentration between 15 and 30 ppm would be the best kind of colloidal silver we should get.
Conclusion
Only colloids of silver which fit all four criteria will truly be safe and effective. Otherwise, safety, effectiveness or both may be in jeopardy. Don’t settle for colloids of silver that meet less than all three of the criteria! Your own well being and those you care about may be harmed by using a low quality colloidal silver that only meets one or two, or less, of the criteria.
Next month we will discuss why these other silver solutions which are not colloidal silver work, but at what expense. Stay tuned!
This post was written by Silver Living Tech. Silver Living Tech specializes in the manufacture and research of colloidal nano particles of silver.
Visit their website at www.silverlivingtech.com


So for it to be considered ‘good/safe’ silver, you want it to glow like the jar on the right? Glow is good?
Yes, Alissa. That is my understanding!
?? do you know how to reduce ionic silver to colloidal silver with a reducing agent under heat? or do you just over-cook with silver wires and batteries? its easily reduced using 1 milligram of vitamin C in water while boiling in a microwave, but filtered through coffee filter first, before that step.
I’m sorry – I don’t know!